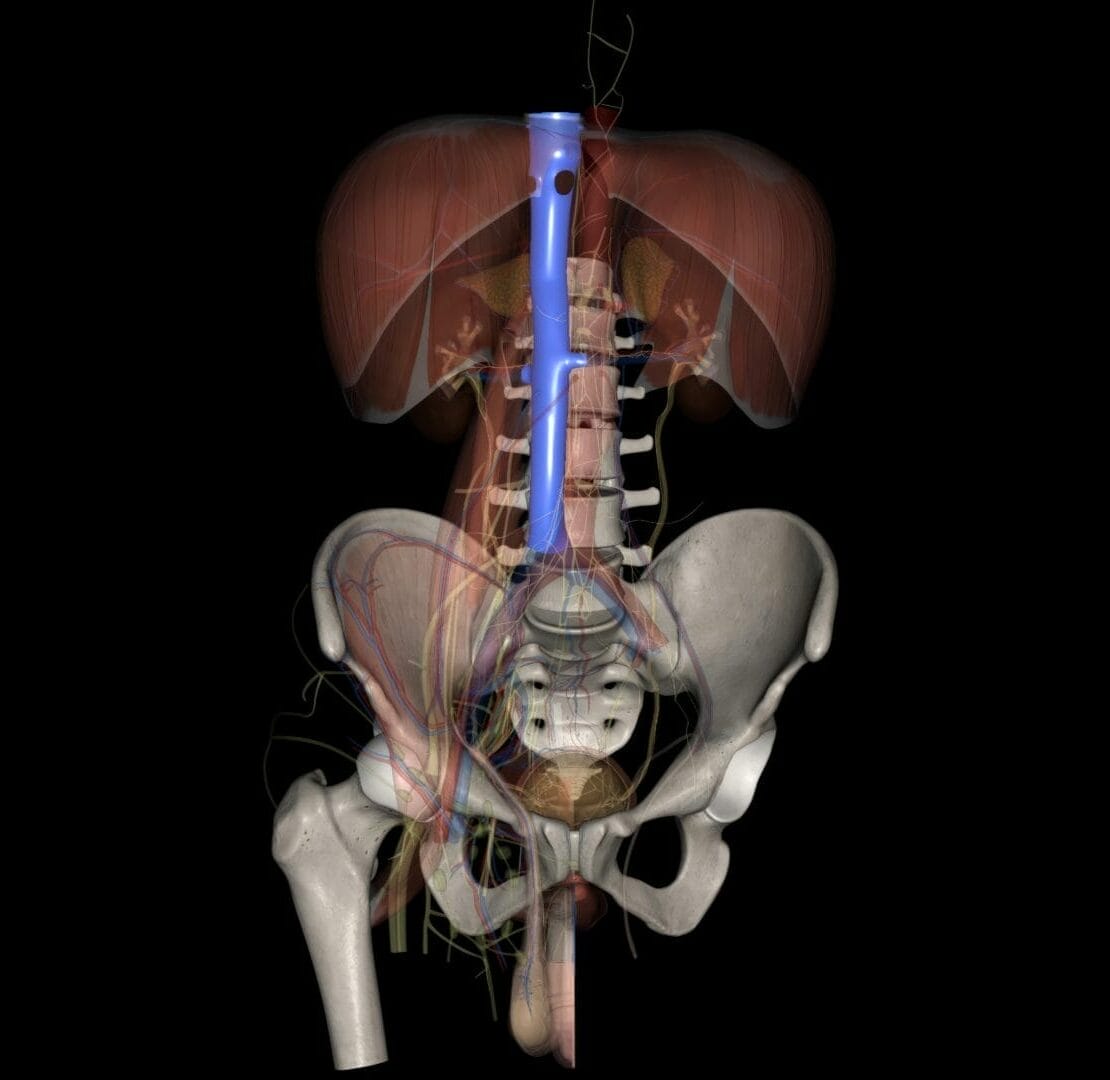
Federal lawsuits filed against
Bard and Cook and other IVC filter manufacturers allege that defects in the design of IVC filters make them more likely to fracture, migrate, tilt or perforate the blood vessel. People who were injured say the companies knew or should have known about the dangers but failed to warn doctors and patients of the risks.
Thousands of IVC filters have been
recalled because they were defective, failed to open, tilted or migrated. In fact, device makers issued seven (7)
IVC filter recalls between 2005 and 2019. Two of these recalls were the serious Class I recalls (the FDA’s most serious/urgent recall) — the 2013 Cordis recall of 33,000 of its OptEast IVC filters and the 2005 Boston Scientific
recall of 18,000 Greenfield filters.
The OptEase recall was to correct labeling and prevent backwards insertion of the devices, and remains the largest recall to date. The last IVC filter recall came in 2019 from Cook, which brought back multiple sets of its Gunter Tulip IVC because of labeling issues.
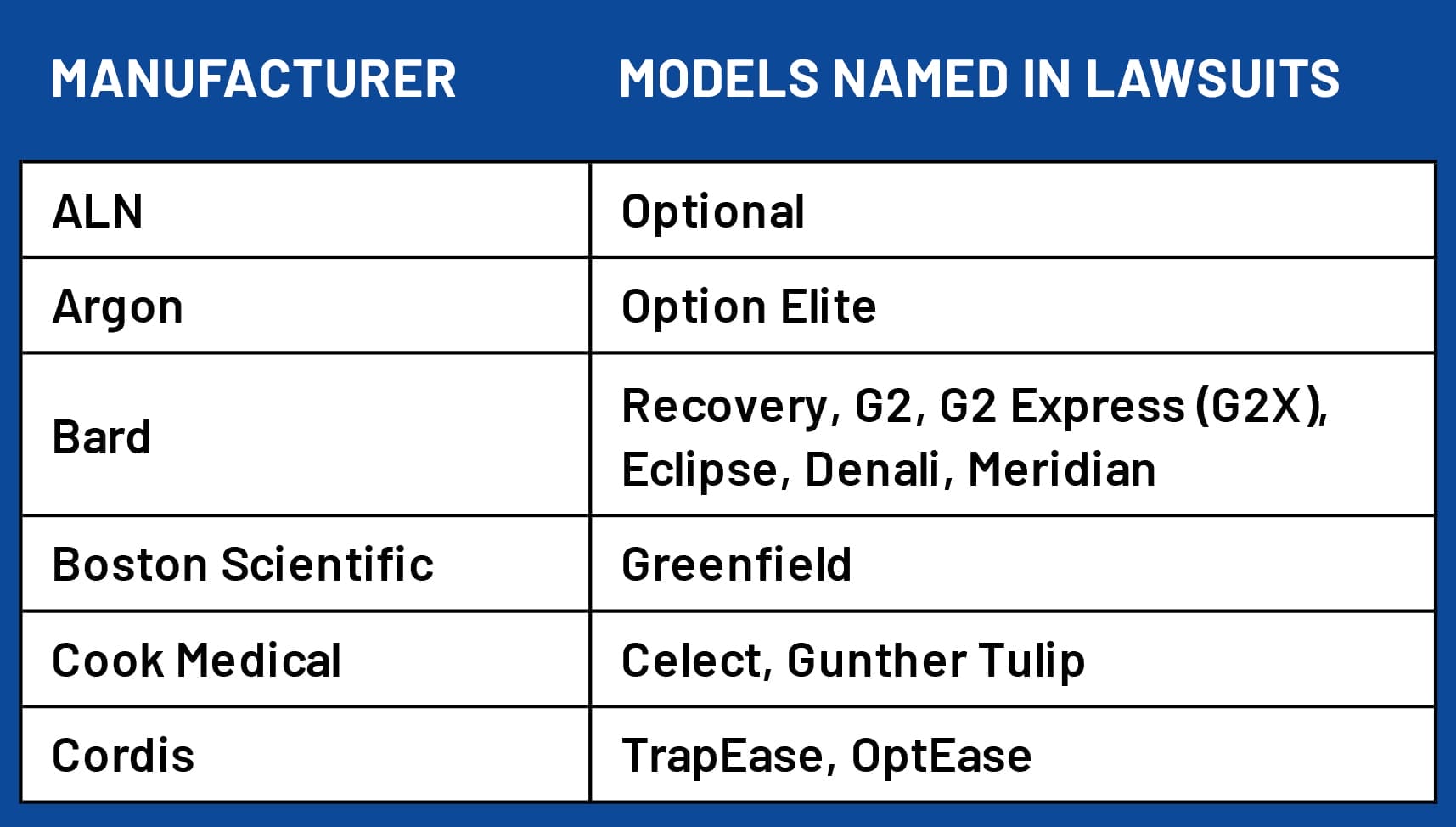
Bard, Cook and Cordis are manufacturers that have multiple models of IVC filters named in legal claims. Cordis and Cook have two models named. Bard has six, according to available filings.